UK approves Pfizer’s Coronavirus vaccine
The United Kingdom becomes the first country to approve a Covid-19 vaccine for use next week
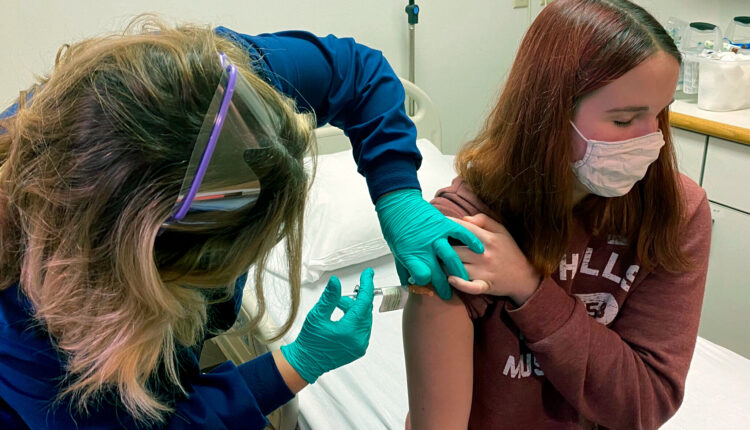
Pfizer and German partner BioNtech have been granted temporary authorization for emergency use for their Covid-19 vaccine in the United Kingdom.
Medicine regulators in the UK have stated that the vaccine, which offers up to 95% against Covid-19, is safe to be rolled out for public use and that the first 800,000 doses will be made available as early as next week in the UK.
In a press release, the CEO of Pfizer Albert Bourla called the emergency use of his company’s vaccine “a historic moment in the fight against Covid-19”
The urgency of the COVID-19 situation has turned the Phizer vaccine into the fastest vaccine to ever go from concept to reality, taking only ten months to follow the same steps that usually take up to ten years.
With the United Kingdom already ordering 40 million doses of the vaccine, enough to immunize 20 million people, this marks the first time citizens outside clinical trials will have access to a COVID-19 vaccine.
How to submit an Op-Ed: Libyan Express accepts opinion articles on a wide range of topics. Submissions may be sent to oped@libyanexpress.com. Please include ‘Op-Ed’ in the subject line.
- Haftar to fly to the US with family, pretending it’s a Libyan official visit - September 13, 2021
- Haftar hires ex-Clinton aide, ex-Republican leader to lobby Washington for Libya elections’ run - September 09, 2021
- Al-Saadi Gaddafi, late dictator’s son, released from Libyan prison - September 06, 2021


